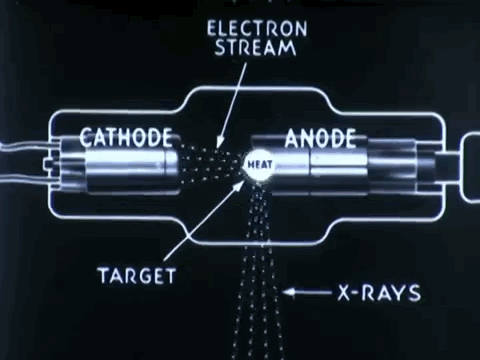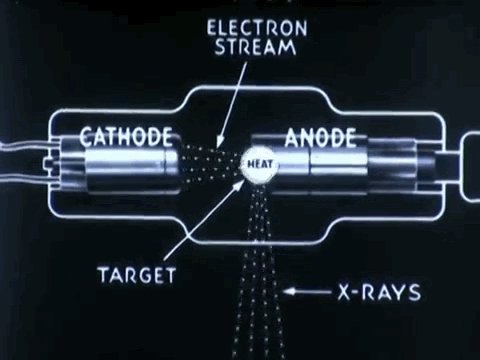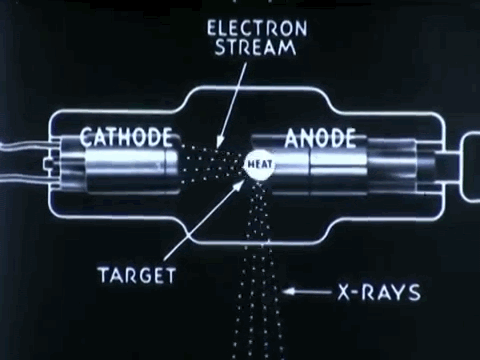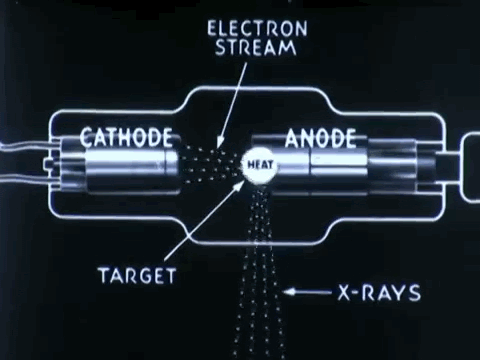
Non-destructive testing is essential to optimize medical device impacts in saving lives
Non-destructive testing (NDT) is used to thoroughly inspect and test products for its flaws, defects, or discontinuiities, without causing any damage to product's original form. NDT procedures can prevent the costs of time, money, and lives, by detecting defects in company products earlier on during its production and approval process.
![OR Technology. (n.d.). Medici Dr Systems [mobile]. OR Technology. Retrieved May 16, 2022, from https://www.or-technology.com/en/products/testing/medici-dr-systems-mobile.html](images/xray.jpg)
![Wilks, S., Katsamenis, O., Carugo, D., Zhang, X., Fader, M., & Keevil, C. (1970, January 1). [PDF] the use of X-ray micro computed tomography (μ-CT) to understand crystalline biofilm blockage in urinary catheters: Semantic scholar. The use of X-Ray micro computed tomography (μ-CT) to understand crystalline biofilm blockage in urinary catheters. Retrieved May 16, 2022, from https://www.semanticscholar.org/paper/The-use-of-X-Ray-micro-computed-tomography-(%CE%BC-CT)-Wilks-Katsamenis/989692d68cc8b3ad296dddcc499d3a0ddbe297a5](images/catheter.png)